Abstract
Background: Despite recent advances, prognosis for pts with NHL who undergo multiple lines of therapy remains poor. Novel drugs that provide durable complete responses (CRs) are needed for these pts. Glofitamab (RG6026) is a novel T-cell-engaging, bispecific antibody that binds bivalently to CD20 on B cells, and monovalently to CD3 on T cells. In study NP30179 (NCT03075696), an ongoing Phase I/II dose-escalation and expansion study, glofitamab fixed-dosing (0.6-25mg) with obinutuzumab pre-treatment (Gpt) achieved high, durable CRs with manageable safety in pts with heavily pre-treated R/R NHL (Dickinson et al. EHA 2020). Step-up dosing (SUD) of glofitamab, in addition to Gpt, allowed dose-escalation up to the highest planned dose of 30mg to maximize efficacy, while mitigating cytokine release syndrome (CRS; [Hutchings et al. J Clin Oncol 2021]). We present updated duration of response (DoR) data from the glofitamab monotherapy fixed-dosing and SUD cohorts of study NP30179 in pts with R/R NHL.
Methods: Pts received 1000mg obinutuzumab 7 days prior to first dose of glofitamab. Glofitamab was given intravenously at a fixed dose (0.6-25mg) every 2 weeks or every 3 weeks (q3w) or with SUD (2.5/10/16mg or 2.5/10/30mg [recommended Phase II dose; RP2D]) on Cycle (C) 1 Day (D) 1 and 8, and then at the target dose from C2D1 q3w, for up to 12 cycles. Response rates are based on Lugano criteria (Cheson et al. J Clin Oncol 2014).
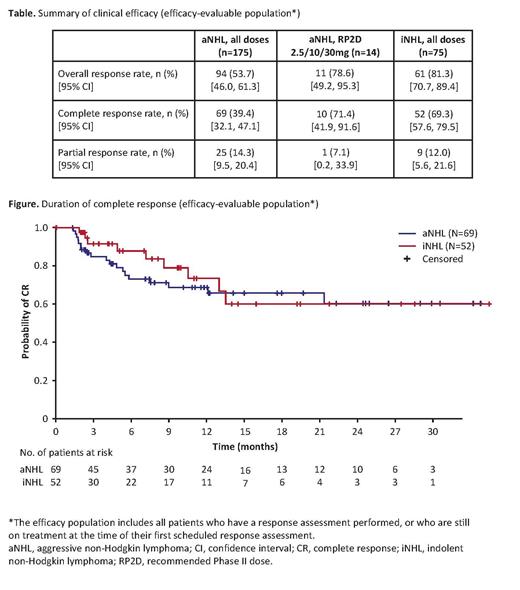
Results: As of May 18, 2021, 258 pts were enrolled in the previously specified cohorts. Median age was 64.0 (range, 22‒86) years, 62.0% were male, and the median number of prior therapies was 3 (range, 1‒12). A total of 183 (70.9%) pts had aggressive NHL (aNHL), and 75 (29.1%) had indolent NHL (iNHL). Of the pts with aNHL, 98 had diffuse large B-cell lymphoma, 26 had mantle cell lymphoma, 31 had transformed follicular lymphoma (FL), and 11 had Richter's transformation. All pts with iNHL had Grade (Gr) 1‒3a FL. Response rates are reported across all doses investigated. Highest responses were seen with the RP2D (2.5/10/30mg) in pts with aNHL (Table). At the clinical cut-off date (CCOD), median duration of follow-up in pts with aNHL was 13.4 (range: 0‒36) months. In efficacy-evaluable pts with aNHL (n=175), the overall response rate (ORR) was 53.7% and the CR rate was 39.4%. Median duration of CR had not yet been reached (95% confidence interval [CI]: 21.4‒not estimable [NE], n=69; Figure); 72.5% of pts with a CR (50/69) were still in CR at the time of analysis. Median DoR (CR plus partial response) was 29.4 months (95% CI: 6.0‒NE; responders, n=94). In pts with iNHL (n=75), ORR was 81.3% and CR rate was 69.3%; median follow-up was 8.6 (range: 0‒37) months. Median duration of CR had not yet been reached (95% CI: 10.5‒NE, n=52; Figure); 82.7% of pts with a CR (43/52) were still in CR at the time of the analysis. Median DoR had not been reached (95% CI: 10.5‒NE; responders, n=61). A total of 149/258 pts (57.8%) experienced a serious adverse event (AE). CRS was the most prevalent AE, occurring in 152/258 pts (58.9%). The majority of CRS events were mild: Gr 1-2, 139 (53.9%) pts; Gr 3, 9 (3.5%) pts; Gr 4, 4 pts (1.6%). Four pts (1.6%) experienced a glofitamab-related AE that led to withdrawal of the study drug. Ninety-two (35.7%) pts experienced a neurological AE; the majority of events were Gr 1 (56/258; 21.7%) or Gr 2 (33/258; 12.8%). Three pts experienced a Gr 3 neurological AE (facial paralysis, syncope, radiculopathy), which were considered unrelated to glofitamab treatment. Immune effector cell-associated neurotoxicity syndrome (ICANS)-like events related to glofitamab occurred in 9 pts (3.5%); all events were Gr 1 or Gr 2, and all but one (Gr 1 tremor) resolved at CCOD.
Conclusions: The current dataset on DoR is the largest presented to date for a CD20xCD3 bispecific antibody, with median follow-up exceeding 13 months for pts with aNHL. Glofitamab, with a fixed treatment duration and 'off-the-shelf' accessibility, has demonstrated high levels of monotherapy activity in heavily pretreated pts with R/R NHL, including those who have received two or more lines of systemic therapy. Glofitamab has shown promising response rates and durable responses a range of different doses for both aNHL and iNHL. Duration of responses in pts with aNHL were in the range of those observed in pts with refractory aNHL from an early chimeric antigen receptor T-cell data set (Neelapu et al. N Engl J Med 2017).
Dickinson: Amgen: Honoraria; Celgene: Research Funding; Takeda: Research Funding; Gilead Sciences: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: travel, accommodation, expenses, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Carlo-Stella: Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Oncology: Honoraria; Celgene: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria; Incyte: Honoraria; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Research Funding. Morschhauser: Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Speakers Bureau; Genentech, Inc.: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZenenca: Membership on an entity's Board of Directors or advisory committees. Patel: BeiGene: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; Fate Therapeutics: Research Funding; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Juno Pharmaceuticals: Consultancy; Kite: Consultancy, Research Funding, Speakers Bureau; MEI Pharma: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; Trillium Therapeutics: Research Funding; Sunesis Pharmaceuticals: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding, Speakers Bureau; Morphosys: Consultancy; Xencor: Research Funding; Curis, Inc: Research Funding; Abbvie: Consultancy; Millenium/Takeda: Research Funding; Velos Bio: Research Funding; Aptevo Therapeutics: Research Funding; AstraZeneca: Consultancy, Research Funding, Speakers Bureau. Khan: Genentech: Research Funding, Speakers Bureau; Astrazeneca: Research Funding, Speakers Bureau; Epizyme: Research Funding, Speakers Bureau; Beigene: Research Funding, Speakers Bureau; Abbvie: Research Funding, Speakers Bureau; Sanofi: Speakers Bureau; Karyopharm: Speakers Bureau; SeaGen: Speakers Bureau; Morphosys: Speakers Bureau; Kite: Speakers Bureau; GSK: Speakers Bureau. Bartlett: Affimed: Research Funding; Autolus: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Forty Seven: Research Funding; Janssen: Research Funding; Kite Pharma: Research Funding; Merck: Research Funding; Millennium: Research Funding; Pharmacyclics: Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Washington University School of Medicine: Current Employment. Iacoboni: BMS/Celgene, Gilead, Novartis, Janssen, Roche: Honoraria. Hertzberg: Roche: Honoraria, Speakers Bureau; MSD: Honoraria; BMS: Honoraria; Takeda: Honoraria; Gilead: Honoraria. Leppä: Genmab: Research Funding; Orion: Consultancy; CHO Pharma USA: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees; University of Helsinki and Helsinki University Hospital: Current Employment; Takeda: Research Funding; Bayer: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Van Den Neste: Novartis: Consultancy, Research Funding; Roche: Research Funding; Celgene: Consultancy. Cartron: Roche, Celgene-BMS: Consultancy; Danofi, Gilead, Novartis, Jansen, Roche, Celgene-BMS, Abbvie, Takeda: Honoraria. Salar: Beigene: Consultancy; BMS/Celgene: Consultancy, Speakers Bureau; EusaPharma: Consultancy; Janssen: Consultancy, Speakers Bureau; Hospital del Mar: Current Employment; Abbvie: Research Funding. Perez-Callejo: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Lundberg: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Relf: F-Star Therapeutics: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Harpoon Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months; Roche Pharmaceutical Ltd: Current Employment, Current equity holder in publicly-traded company. Clark: Roche Products Ltd: Current Employment. Humphrey: Roche: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Hutchings: Novartis: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Janssen: Honoraria, Research Funding; Genmab: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Genentech: Honoraria, Research Funding.
Glofitamab is a full-length, humanized immunoglobulin G1 bispecific antibodywith a 2:1 molecular format that facilitates bivalent binding to CD20 on B-cells, and monovalent binding to CD3 on T-cells. Glofitamab redirects T cells to engage and eliminate malignant B cells. Glofitamab is an investigational agent.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal